Clinical Research for Cosmetics Efficacy Study Team
To safety and efficacy inspection of cosmetics ingredients from antibacterial evaluation for functional cosmetics and quasi-drugs!
We progress and analyze non-clinical and clinical experiments by one-step. We proceed the evaluation of feeling of use and of degree of preference in every evaluation service. The test results can be submitted to KFDA.
IRB (Institutional Review Board) means a clinical experiment judging committee that is established independently in the institute in order to guarantee the safety and right of the person under test from a research with object of human body. KIDS (Korea Institute of Dermatological Science) organizes IRB with members in ethical area, scientific area and medical area, and they confirm the thorough verification procedure about the whole research.
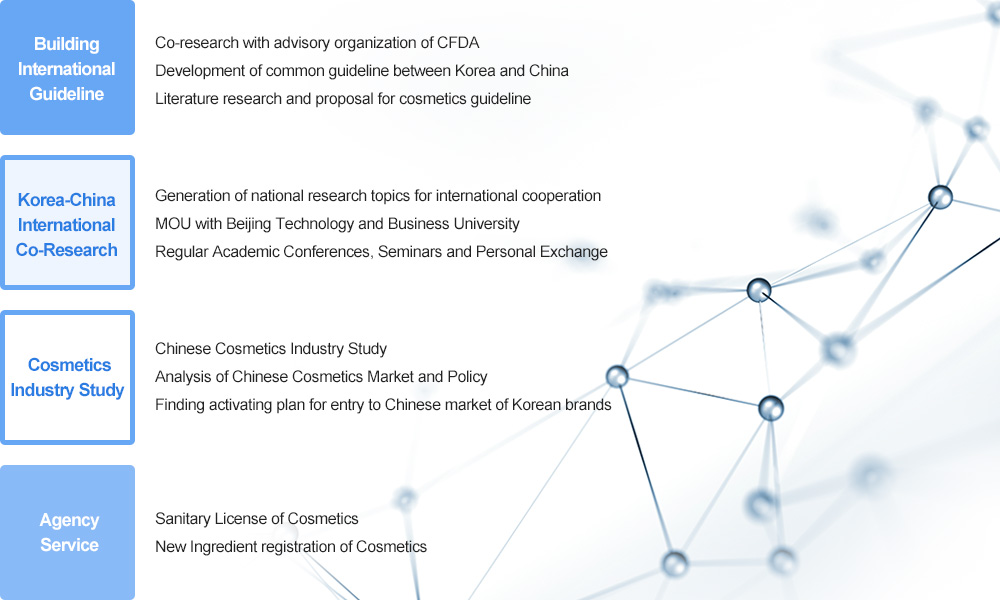
Introduction of Korean-Chinese Cosmetics Industry Study Team
The Korean-Chinese Cosmetics Industry Study Team of GENECELLPHARM plays a role of bridgehead of cosmetics industries of both countries by consistently understanding and quickly coping with the Chinese cosmetics industry and trend of policy. And also we help to get smooth and fast permission and registration by doing business for documentation and screening for registration of new ingredient of cosmetics and issue of sanitary license through cooperation with a local company.
Sanitary License of CFDA?
CFDA (China Food & Drug Administration) is an affiliated organization of Chinese State Council and has a similar function with KFDA. It has a responsibility of administration and supervision to food, cosmetics and medical equipment. The Sanitary License of CFDA is the registering process and the license in order for a foreign brand to export the relevant product to China. At present China requires Sanitary Licenses to all imported cosmetics.
Introduction of Animal Replacement Experiment Study Team
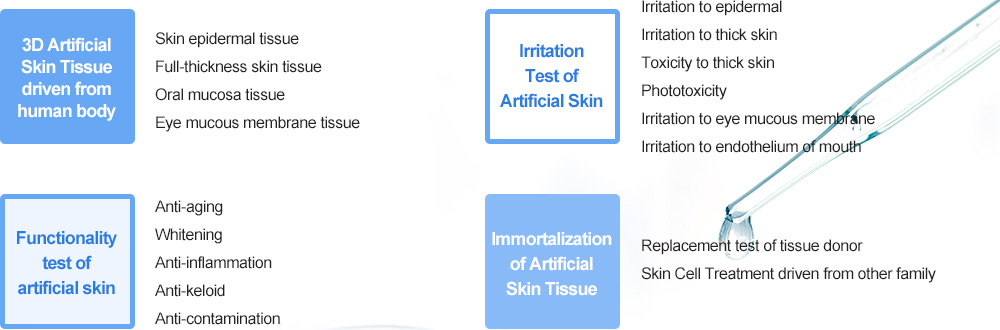
The Animal Replacement Experiments are fulfilled in the cosmetics market of Europe as the center. The Animal Replacement Experiment applies 3R principles of animal welfare perspective to animal experiment. The world’s cosmetics market has already carried out the Animal Replacement Experiment, and the domestic cosmetics market also fulfills the Animal Replacement Experiment. 3D Skin Tissue Culture is an Animal Replacement Experiment with concern because it can verify skin permeability, toxicity and inflammatory reaction even to a finished good as well as an ingredient.
GENECELLPHARM carries out the safety and efficacy evaluation of cosmetics and drugs by utilizing artificial skin tissues and linking the results of application test to human body together according to “OECD GUIDELINE OF ANIMAL REPLACEMENT EXPERIMENT” based on Laboratory Animal Act announced by MFDS.
동물대체 시험법
| Guideline classification of Animal Replacement Experiment |
Title |
| Single dosage injection toxicity testing |
Skin toxicity test using artificial epidermal tissue |
| Skin irritation test |
Skin irritation test using artificial epidermal tissue |
| Phototoxicity test |
Phototoxicity test using artificial melanin epidermal tissue |
| Irritation to eye mucous membrane test |
Irritation to eye mucous membrane test using artificial cornea tissue |
| Irritation to mouth test |
Mouth irritation test using artificial mouth tissue |
Live animals are used for testing of safety and efficacy of cosmetics or spreading drugs. But this method is very brutal and shows big difference from results to human skin. Therefore lately the interest and demand about animal replacement experiment have increased over the world. The 3D skin tissue and immortalized 3D skin tissue developed by Korea Institute of Dermatological Science recreate almost similarly to skin tissues of humankind. They can be used for evaluating safety and efficacy with more accuracy as well as replacing inhumane animal experiment.
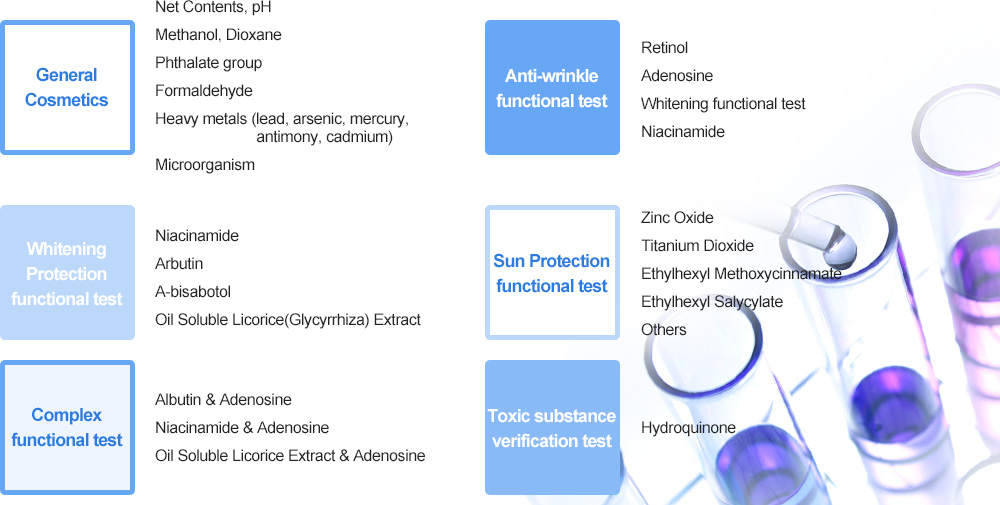
Introduction of Quality Inspection Study Team
Quality Inspection Study Team is an analyzing institute of standard analysis technology for cosmetics contributing the quality and safety of domestic and international cosmetics under the Cosmetics Act, its enforcement ordinance and guideline officially announced by KFDA. This is composed of equipment with high performance according to advancement in scientific technology and of researchers leading the up-to-date analysis technology.
- Reiabiity
- We progress all analysis jobs under the test guidelines proposed by KFDA
- Accuracy
- We produce exact results of analysis by using the up-to-date analysis equipment.
- Professionalism
- The study is carried out by analysis technology researchers and cosmetics researchers together.
We propose the opinion of product through diverse analysis.
- Rapidity
- We progress with speed from inquiry of test to production of output by using the organized system.